Step-by-step explanation:
The given symbol is as follows
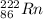 . The number written at the top left represents mass number of radon whereas number written on the bottom left represents the atomic number.
. The number written at the top left represents mass number of radon whereas number written on the bottom left represents the atomic number.
Mass number is the sum of total number of protons and neutrons present in an atom. On the other hand, atomic number is the number of protons.
Therefore, in the given radon atom there are 86 protons.
Calculate the number of neutrons as follow.
No. of neutrons = Mass number - no. of protons
= 222 - 86
= 136
It is known that in a neutral atom, number of protons equal number of electrons. Thus, number of electrons in radon atom is 86.
Hence, we can conclude that in the given atom the numbers are as follows.
- 86 protons.
- 136 neutrons.
- 86 electrons.