Hello!
- The volume of a gas-filled balloon is 30.0 L at 40 °C and 153 kPa pressure. What volume will the balloon have at standard temperature and pressure (273.15 K and 101.3 kPa)?
a. 17.3 L
b. 23.7 L
c. 39.5 L
d. 51.9 L
We have the following data:
V1 (initial volume) = 30 L
T1 (initial temperature) = 40ºC (in Kelvin)
TK = TºC + 273.15
TK = 40 + 273.15 → T1 (initial temperature) = 313.15 K
P1 (initial pressure) = 153 kPa
V2 (final volume) = ? (in L)
T2 (final temperature) = 273.15 K
P2 (final pressure) = 101.3 kPa
Now, we apply the data of the variables above to the General Equation of Gases, let's see:
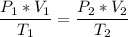
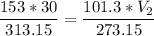
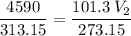
multiply the means by the extremes
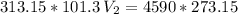
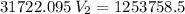
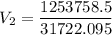

Answer:
c. 39.5 L
_______________________
_______________________
- A gas that has a volume of 28 liters, a temperature of 45 °C, and an unknown pressure, has its volume increased to 34 liters and its temperature decreased to 35 °C. If I measure the pressure after the change to be 2.0 atm, what was the original pressure of the gas?
a. 1.5 atm
b. 1.7 atm
c. 2.8 atm
d. 2.5 atm
We have the following data:
V1 (initial volume) = 28 L
T1 (initial temperature) = 45ºC (in Kelvin)
TK = TºC + 273.15
TK = 45 + 273.15 → T1 (initial temperature) = 318.15 K
P1 (initial pressure) = ? (in atm)
V2 (final volume) = 34 L
T2 (final temperature) = 35ºC (in Kelvin)
TK = TºC + 273.15
TK = 35 + 273.15 → T2 (final temperature) = 308.15 K
P2 (final pressure) = 2 atm
Now, we apply the data of the variables above to the General Equation of Gases, let's see:
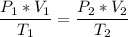
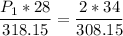
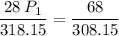
multiply the means by the extremes
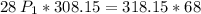
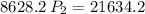
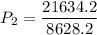
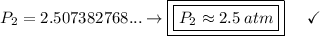
Answer:
d. 2.5 atm
_______________________