Answer : The mass of iron metal produced will be, 61.376 grams.
Solution : Given,
Molar mass of iron(II)nitrate = 179.85 g/mole
Molar mass of Fe = 56 g/mole
First we have to calculate the mass of iron(II)nitrate.
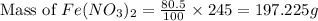
Now we have to calculate the moles of iron(II)nitrate.
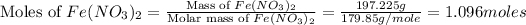
Now we have to calculate the moles of iron metal.
The given balanced reaction is,
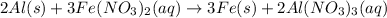
From the balanced reaction, we conclude that
As, 3 moles of iron(II)nitrate react to give 3 moles of iron metal
So, 1.096 moles of iron(II)nitrate react to give
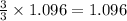 moles of iron metal
moles of iron metal
Now we have to calculate the mass of iron metal.
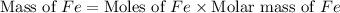

Therefore, the mass of iron metal produced will be, 61.376 grams.