Answer: The equilibrium lies far to the right so that products are very heavily favored.
Step-by-step explanation:
Strong acids are defined as acids with high concentration of
 ion in their aqueous solution. They get easily dissociate in their aqueous solution.
ion in their aqueous solution. They get easily dissociate in their aqueous solution.
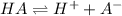
![K_a=([H^+][A^-])/([HA])](https://img.qammunity.org/2018/formulas/chemistry/high-school/sbyfrky03tp12nb6zjk5fz8u7ve2g4w8q6.png)
Generally, value of K_a of strong acids are greater than 1.
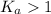
![K_a>([H^+][A^-])/([HA])](https://img.qammunity.org/2018/formulas/chemistry/high-school/74sak1eff7rwpwmlmzg1v4xgupet189lp8.png)
So, to satisfy this above condition concentrations:
![[HA]<[H^+]* [A^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/jvdkccmw285l05oxf48xm6n74brzmtig32.png)
Hence, the correct answer is :The equilibrium lies far to the right so that products are very heavily favored.