Answer : The amount of sugar crystallized out of the saturated solution when it cooled to
 is, 147 grams.
is, 147 grams.
Solution :
First we have to calculate the amount of sugar remains in the solution.
As, 100 gram of water contains 203.9 gram of sugar
So, 75 gram of water contains
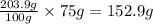 of sugar
of sugar
Now we have to calculate the amount of sugar crystallized out of the saturated solution.
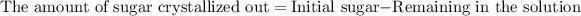
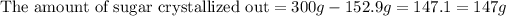
Therefore, the amount of sugar crystallized out of the saturated solution when it cooled to
 is, 147 grams.
is, 147 grams.