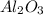Answer : The correct formula for a compound formed from the elements Al and O is,
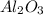
Explanation :
Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
As we know that the aluminum is a metal and has 3 valence electron and oxygen is a non-metal and has 6 valence electrons.
When aluminum atom donate electrons to oxygen atom then it forms an ionic compound that is aluminum oxide.
Aluminum oxide is an ionic compound. It is made up of two different ions which are aluminum ion
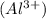 and oxide ion
and oxide ion
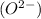 by the criss-cross method. Thus, the correct chemical formula for aluminum oxide is
by the criss-cross method. Thus, the correct chemical formula for aluminum oxide is
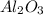 .
.
Hence, the correct formula for a compound formed from the elements Al and O is,