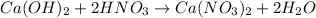Step-by-step explanation:
The reaction between calcium hydroxide and nitric acid is as follows.
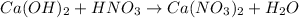
Number of reactant atoms are as follows.
Number of product atoms are as follows.
To balance the given chemical equation, multiply
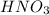 by 2 on reactant side and multiply
by 2 on reactant side and multiply
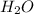 by 2 on the product side.
by 2 on the product side.
Therefore, the balanced chemical equation will be as follows.