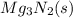Answer: The product of the given reaction is
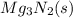
Step-by-step explanation:
When magnesium solid reacts with nitrogen gas, it leads to the formation of an ionic solid known as magnesium nitride.
The chemical equation for the reaction of magnesium and nitrogen gas follows:
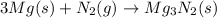
By Stoichiometry of the reaction:
3 moles of magnesium solid reacts with 1 mole of nitrogen gas to produce 1 mole of magnesium nitride.
The given reaction is considered as synthesis reaction.
Hence, the product of the given reaction is