Answer: 8 moles
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 Liters at STP and contains avogadro's number
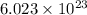 of particles.
of particles.
1 molecule of
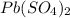 contains = 8 oxygen atoms.
contains = 8 oxygen atoms.
Thus
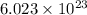 molecules of
molecules of
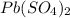 contains =
contains =
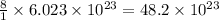 atoms of Oxygen.
atoms of Oxygen.
To calculate the moles, we use the equation:
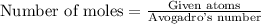
![\text{Number of moles}=\frac{48.2* 10^(23)}{6.023* 10^(23)=8moles]()
Thus 1 mole of
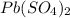 contains 8 moles of oxygen atoms.
contains 8 moles of oxygen atoms.