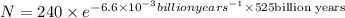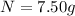Answer: 7.50 grams of radioactive isotope will remain
Explanation:- Radioactive decay follows first order kinetics
Half-life of sample of lanthanum-138 = 105 billion years
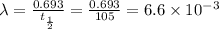
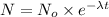
N = amount left after time t = ?
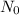 = initial amount = 240 g
= initial amount = 240 g
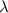 = rate constant =
= rate constant =
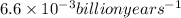
t= time = 525 billion years
Putting in the values, we get