Step-by-step explanation:
The given data is as follows.
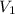 = 20 liter,
= 20 liter,
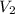 = ?
= ?
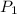 = 150 atm,
= 150 atm,
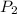 = 1.00 atm
= 1.00 atm
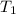 = 27 + 273 = 300 K,
= 27 + 273 = 300 K,
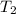 = 37 + 273 = 310 K
= 37 + 273 = 310 K
Therefore, calculate the value of
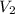 as follows
as follows
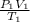 =
=
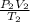
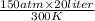 =
=
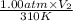
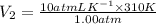
= 3100 liter
Therefore, we can conclude that the volume of the balloon is 3100 liter.