Answer : The number of ions produced in the dissociation of the compound BaS are, two.
Explanation :
Dissociation reaction : It is defined as the chemical reaction in which a compound breaks into two or more ions.
The general representation of reaction is :
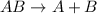
The dissociation reaction of the given compound will be,
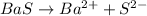
From the reaction we conclude that, there are two ion are produced in the dissociation of BaS compound which are,
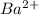 ion and
ion and
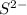 ion.
ion.
Hence, the number of ions produced in the dissociation of the compound BaS are, two.