Answer : The element 'Xenon' belongs to the class of noble gas.
Explanation :
The element 'Xenon' with symbol 'Xe' belongs to group 18. The atomic number of xenon is, 54. It is a colorless, odorless noble gas which is found in the earth's atmosphere.
The electronic configuration of xenon (Xe) will be,
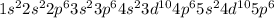
Hence, the element 'Xenon' belongs to the class of noble gas.
The element xenon are shown by the red box in the periodic table.