Answer:
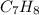
Step-by-step explanation:
Hello,
In this case, as toluene just have carbon and hydrogen, the moles coming from the 4.69mg of carbon dioxide match with the carbon moles contained into the toluene as well as for the hydrogen but for the 1.10mg of water which has two hydrogens inside, as shown below:

Now, we divide both carbon and hydrogen moles by the carbon moles to get the number relating them as follows:
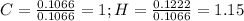
Finally, the factor multiplying such relations suitable to get whole numbers turns out being seven (7), therefore, toluene's empirical formula is:
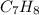
Best regards.