The equation is:
P ( t ) = A * (1/2 )^(t/h)
If a sample contains 18% of the original amount of Radon - 222 and h = 3.8:
0.18 * A = A * ( 0.5 )^(t/3.8) / : A ( we will divide both sides of the equation by A )
0.18 = ( 0.5 )^(t/3.8)
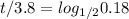
t / 3.8 = 2.47 ≈ 2.5
t = 3.8 * 2.5 = 9.5
Answer:
The best estimate for the age of the sample is 9.5 days.