Answer:
The pressure must be applied at 450 K should be 1,125 torr.
Step-by-step explanation:
At constant volume of 200 mL
Initial pressure exerted by the gas =
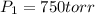
Initial temperature of the gas =
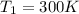
Final pressure exerted by the gas =
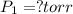
Final temperature of the gas =
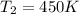
Applying Gay Lussac Law:
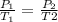
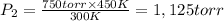
The pressure must be applied at 450 K should be 1,125 torr.