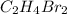Answer : The molecular of the compound is,
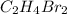
Explanation :
The Empirical formula =
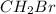
The empirical formula weight = 1(12) + 2(1) + 1(80) = 94 gram/eq
Now we have to calculate the molecular formula of the compound.
Formula used :
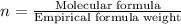
where,
n = valency factor
Now put all the given values in this formula, we get:
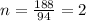
Molecular formula =
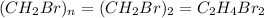
Therefore, the molecular of the compound is,