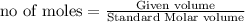Answer:
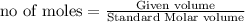
Explanation:
According to Avogadro's law, 1 mole of every gas occupies 22.4 L of volume at standard temperature and pressure conditions.
Thus Standard molar volume of substance= 22.4 L
To calculate the moles from mass ,we use the formula:
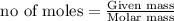
To calculate the moles from volume ,we use the formula:
Similarly,