The property that relates the number of moles of an atom and the mass of the same atom is the atomic weight.
The atomic weight can be consulted in a periodic table.
Consulting one, the atomic weight of He is 4.0026 g/mol.
We can then use rule of three to find the answer:
In 4.0026 g of He, we have 1 mol of He, thus, in 23g of He we have x moles of He:
4.0026 g --- 1 mol
23 g --- x
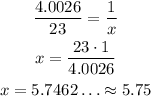
Thus, in 23 g of He, there is approximately 5.75 moles.