Answer: A-they are the same element
Explanation: An element is characterized by its atomic number or the number of protons.
Two atoms having the same number of protons but different numbers of neutrons will have same atomic number but different mass number.Thus they are isotopes of the same element.
As Atomic number = Number of protons
Mass number = number of protons + number of neutrons
For example:
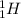 and
and
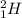 are both isotopes of Hydrogen with same number of protons but different number of neutrons.
are both isotopes of Hydrogen with same number of protons but different number of neutrons.