Answer
V2 = 738.57 mL
Step-by-step explanation
Given
Volume 1 = 735 mL
Pressure 1 = 1.20 atm
Temperature 1 = 112 ∘C = 385 K
Pressure 2 = 648 mmHg = 0.853 atm
Temperature 2 = 275 K
Required: Volume 2 in mL
Solution:
Use a combined gas law
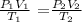
V2 = (P1V1T2)/T1P2
V2 = (1.20 x 735 x 275)/(385 x 0.853)
V2 = 738.57 mL