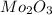Answer : The empirical formula is,
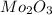
Solution :
First we have to calculate the mass of molybdenum and mass of oxygen.
Mass of molybdenum = Mass of crucible and molybdenum - Mass of crucible
Mass of molybdenum (Mo) = 39.52 - 38.26 =1.26 g
Mass of oxygen = Mass of crucible and molybdenum oxide - Mass of crucible and molybdenum
Mass of oxygen (O) = 39.84 - 39.52 =0.32 g
Molar mass of Mo = 96 g/mole
Molar mass of O = 16 g/mole
Step 1 : convert given masses into moles.
Moles of Mo =
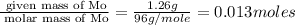
Moles of O =
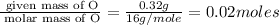
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For Mo =
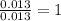
For O =
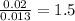
The ratio of Mo : O = 1 : 1.5
In the whole number the ratio of Mo : O = 2 : 3
The mole ratio of the element is represented by subscripts in empirical formula.
Therefore, the empirical formula is,