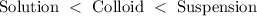Answer: Order of mixtures having particle size smallest to large is:
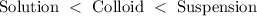
Step-by-step explanation:
The solutions are classified into 3 types on the basis of size of the particles.
1.) Solution: When the particle size is between 0.1 nm to 1 nm, then the solution is considered as a true solution.
2,) Colloid: When the particle size is between 2 to 1000 nm, then the solution is considered as a colloid.
3.) Suspension: When the particle size is greater than 1000 nm, then the solution is considered as a suspension.
Hence, the order of mixtures having particle size smallest to large is: