Answer : The volume of
 produced will be, 48 liters.
produced will be, 48 liters.
Explanation : Given,
Volume of
 = 60 L
= 60 L
First we have to calculate the moles of
 .
.
The given balanced chemical reaction is,
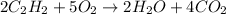
From the balanced chemical reaction, we conclude that
As, 22.4 L volume of
 present in 1 mole of
present in 1 mole of

So, 60 L volume of
 present in
present in
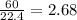 mole of
mole of

Now we have to calculate the moles of
 .
.
From the reaction we conclude that,
As, 5 moles of
 react to give 4 moles of
react to give 4 moles of

So, 2.68 moles of
 react to give
react to give
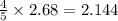 moles of
moles of

Now we have to calculate the volume of
 .
.
At STP,
As, 1 mole of
 contains 22.4 L volume of
contains 22.4 L volume of

So, 2.144 mole of
 contains
contains
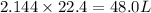 volume of
volume of

Therefore, the volume of
 produced will be, 48 liters.
produced will be, 48 liters.