Answer:
Barium-139
Step-by-step explanation:
initial mass of the sample at 12:02:00 PM is given as
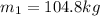
Mass of the sample at 4:11:00 PM is given as
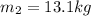
so here since it is a sample of radioactive isotope so here we will say
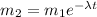
now from above data we know that the time taken to reduce the mass is

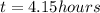
now from above equation we will have
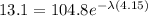
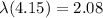
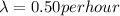
so here half life is given as
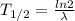

So here this isotope must be the one which will have half life of 1.4 hours
So it is nearly Barium-139