Answer: copper chloride
Explanation:

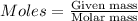
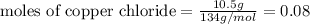

From the balanced equation. it can be seen that 3 moles of copper chloride reacts with 2 moles of Al.
Thus 0.08 moles of copper chloride reacts with=
 moles of Al.
moles of Al.
Thus copper chloride is the limiting reagent as it limits the formation of product and aluminium is the excess reagent as it is left unused. (0.46-0.05)=0.41 moles are in excess.