Answer: Option (d) is the correct answer.
Step-by-step explanation:
Group 2 elements are also known as alkaline earth metals and they are rich in electrons.
For example, calcium is a group 2 element with atomic number 20 and its electronic configuration is 2, 8, 2. In order to gain stability, calcium loses to electrons and becomes
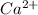 ion.
ion.
Similarly, all the elements of group 2 donates electrons in order to form ionic bonds.
Hence, we can conclude that all group 2 elements have in common that they tend to form ionic bonds by losing electrons.