The empirical formula of the compound is Al2O3.
The empirical formula indicates the minimum amount of each element in a compound, and does not necessarily matches with its molecular formula.
Since the compound is made up of aluminium and oxygen, we need to calculate the moles of each element, that is the subscripts (x,y) of the compound AlxOy.
1st) It is necessary to divide the percentage of each element by its mass:
- Aluminium:
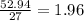
- Oxygen:
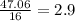
2nd) Now we have to divide both results by the smallest number, that is 1.96:
- Aluminium:
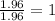
- Oxygen:
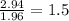
3rd) Since in this case the subscript for the oxygen atom is not a whole number, it is necessary to multiply the values obtained by the smallest number that converts them into a wholw number, in this case it must be multiplied by 2:
- Aluminium:
1x2=2
- Oxygen:
1.5x2=3
So, the empirical formula of the compound is Al2O3.