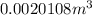Answer : The correct option is, (A)
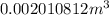
Solution : Given,
Volume of mercury at
 is
is
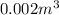
As mercury is a liquid. So, we have to apply the volume of expansion of liquid.
Formula used for the volume expansion of liquid,
![V_(T)=v_(1)[1+\gamma (T_(2)-T_(1))]](https://img.qammunity.org/2018/formulas/physics/high-school/vmzog0xvnwtirceud73zw08hwlbnl7yyyk.png)
or,
![V_(2)=V_(1)[1+\gamma (T_(2)-T_(1))]](https://img.qammunity.org/2018/formulas/physics/high-school/cg3j1ado2yuepo2tgviot5q3bm7klus4p1.png)
where,
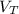 = volume of liquid at temperature
= volume of liquid at temperature
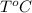
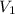 = volume of liquid at temperature
= volume of liquid at temperature

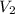 = volume of liquid at temperature
= volume of liquid at temperature
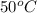
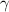 = volume expansion coefficient of mercury at
= volume expansion coefficient of mercury at
 is 0.00018 per centigrade
is 0.00018 per centigrade
Now put all the given values in the above formula, we get
![V_(2)=0.002[1+0.00018(50-20)]=0.0020108m^3](https://img.qammunity.org/2018/formulas/physics/high-school/leu730k352s8oshig6xwecg0hve3re1uuu.png)
Therefore, the volume of mercury at
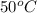 is,
is,