Answer:
27 g/mol
Step-by-step explanation:
To calculate the molar mass of element X, we need to calculate the mass in a given number of moles.
- The moles of X can be calculated by using the ratio of O atoms to X atoms in the molecule:
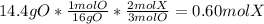
- The mass of X₂O₃ that is not oxygen, is element X. In other words, the mass of the atoms of the X element contained in the 30.6 g sample is:
30.6 g - 14.4 g = 16.2 g of X
Thus, the molar mass of element X is
16.2 g / 0.60 mol = 27 g/mol