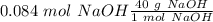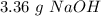Answer:
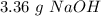
Step-by-step explanation:
We have to start with the molarity equation:
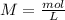
Where:
M= 0.35
L=0.24
(We have to remember that we need to convert the mL into L using the conversion ratio 1 L = 1000 mL). Then with these two values we can calculate the moles of NaOH on this solution, so:
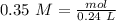
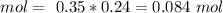
The next step is the conversion from mol to grams. To do this we have to calculate the molar mass of NaOH. So, we have to find the atomic mass of each atom in the compound, so:
Na => 23 g/mol
O => 16 g/mol
H => 1 g/mol
When we add all these values we will have a value of 40 g/mol for NaOH. Now, we can calculate the grams in the 0.084 mol of NaOH: