pH measures the concentration of hydrogen ions on the log 10 scale. Thus, a pH 2 solution has 3 order of magnitude difference from that of a solution of pH of 5.
Therefore,
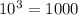
. A solution of pH 5 has 1000 times more hydrogen ions than that in a solution with a pH of 5.