Step-by-step explanation:
A hydrocarbon is defined as a specie which contains atoms of carbon and hydrogen.
For example,
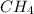 is a hydrocarbon.
is a hydrocarbon.
When there are only single bonds present in a hydrocarbon then it is known as a saturated hydrocarbon.
When a hydrocarbon contains a double or triple bond then it is known as an unsaturated hydrocarbon.
For example,
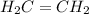 is an unsaturated hydrocarbon.
is an unsaturated hydrocarbon.
Therefore, we can conclude that the statement all of the atoms of a saturated hydrocarbon are bonded by 1 or 2 pair(s) of electrons, is not true.