Answer : The correct option is, (B) sodium and potassium
Explanation :
Valence electrons : Valence electrons are those electrons which are present in the outermost shell of the element.
As we know that,
Option (A) :
Element = Hydrogen
Atomic number = Number of electrons = 1
The electronic configuration is,
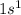
The number of valence electrons = 1
Element = Helium
Atomic number = Number of electrons = 2
The electronic configuration is,
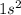
The number of valence electrons = 2
Option (B) :
Element = Sodium
Atomic number = Number of electrons = 11
The electronic configuration is,
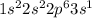
The number of valence electrons = 1
Element = Potassium
Atomic number = Number of electrons = 19
The electronic configuration is,
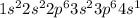
The number of valence electrons = 1
Option (C) :
Element = Carbon
Atomic number = Number of electrons = 6
The electronic configuration is,
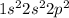
The number of valence electrons = 4
Element = Oxygen
Atomic number = Number of electrons = 8
The electronic configuration is,
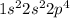
The number of valence electrons = 6
Option (D) :
Element = Boron
Atomic number = Number of electrons = 5
The electronic configuration is,
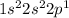
The number of valence electrons = 3
Element = Beryllium
Atomic number = Number of electrons = 4
The electronic configuration is,
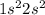
The number of valence electrons = 2
From this we conclude that, the sodium and potassium are the elements have the same number of valence electrons.
Hence, the correct option is, (B)