The question is incomplete, here is a complete question.
Which formula represents a compound that is formed primarily by sharing electrons?
1. KCl
2. CaCl₂
3. CrCl₃
4. CCl₄
Answer : The correct option is, (4) CCl₄
Explanation :
Covalent compound : It is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound.
The covalent compound are usually formed when two non-metals react.
Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
(1) KCl
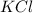 is an ionic compound because potassium element is a metal and chlorine element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
is an ionic compound because potassium element is a metal and chlorine element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
(2) CaCl₂
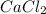 an ionic compound because calcium element is a metal and chlorine element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
an ionic compound because calcium element is a metal and chlorine element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
(3) CrCl₃
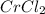 an ionic compound because chromium element is a metal and chlorine element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
an ionic compound because chromium element is a metal and chlorine element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
(4) CCl₄
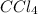 is a covalent compound because sharing of electrons takes place between carbon and chlorine. Both the elements are non-metals and hence, will form covalent bond.
is a covalent compound because sharing of electrons takes place between carbon and chlorine. Both the elements are non-metals and hence, will form covalent bond.
Hence, the formula represents a compound that is formed primarily by sharing electrons is, CCl₄