Answer : The limiting reagent is, NaOH
Explanation : Given,
Mass of HCl = 30 g
Mass of NaOH = 20 g
Molar mass of HCl = 36.5 g/mole
Molar mass of NaOH = 40 g/mole
First we have to calculate the moles of
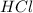 and
and
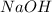 .
.
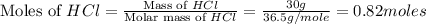
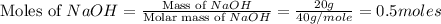
Now we have to calculate the limiting and excess reagent.
The balanced chemical reaction is,

From the balanced reaction we conclude that
As, 1 mole of
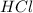 react with 1 mole of
react with 1 mole of
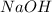
So, 0.82 moles of
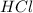 react with 0.82 moles of
react with 0.82 moles of
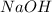
That means, in the given balanced reaction,
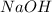 is a limiting reagent because it limits the formation of products and
is a limiting reagent because it limits the formation of products and
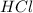 is an excess reagent.
is an excess reagent.
Hence, the limiting reagent is NaOH