Answer: B.
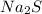
Explanation: An ionic bond is formed when an element completely transfers its valence electron to another element. Ionic bond is formed between a metal and an non-metal.
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Here
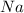 is having an oxidation state of +1 and sulfur has an oxidation state of -2. Thus they combine to form neutral
is having an oxidation state of +1 and sulfur has an oxidation state of -2. Thus they combine to form neutral
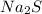 .
.
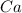 is having an oxidation state of +2 and fluorine has an oxidation state of -1. Thus they combine to form neutral
is having an oxidation state of +2 and fluorine has an oxidation state of -1. Thus they combine to form neutral
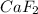 . and not
. and not
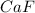 .
.