Answer: The coefficient of Fe(l) is 2
Step-by-step explanation:
Balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side will be equal to the total number of individual atoms on the product side. These equations follow law of conservation of mass.
The balanced chemical equation for the given reaction follows:
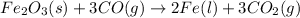
By Stoichiometry of the reaction:
1 mole of iron (III) oxide reacts with 3 moles of carbon monoxide to produce 2 moles of iron metal and 3 moles of carbon dioxide.
Hence, the coefficient of Fe(l) is 2