Answer : The volume of methane gas needed are 4.25 liters.
Explanation :
The balanced chemical reaction is:
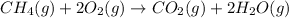
At STP, 1 mole of substance contains 22.4 L volume of gas.
From the given reaction we conclude that,
1 mole of methane react to give 2 moles of water vapor.
As,
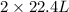 volume of water vapor produced from 22.4 L volume of methane gas
volume of water vapor produced from 22.4 L volume of methane gas
So, 8.5 L volume of water vapor produced from
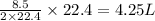 volume of methane gas
volume of methane gas
Therefore, the volume of methane gas needed are 4.25 liters.