Answer: Li percentage in
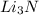 is 59.8%.
is 59.8%.
Explanation: The question asks to calculate the mass percentage of Li in
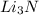 .
.
The formula used for mass percentage of an element in a compound is:
mass percentage =
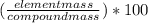
Atomic mass of Li is 6.94 and atomic mass of N is 14.0.
There are three Li in the given compound. So, the mass of Li in the compound is = 3(6.94) = 20.82
Mass of compound = 20.82 + 14.0 = 34.82
Mass percentage of Li =
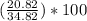 = 59.8%
= 59.8%
Hence, the percentage of Li in
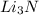 is 59.8%.
is 59.8%.