Answer : The correct option is, (B) molality of solution
Explanation :
Elevation in boiling point : It is a colligative property in which the magnitude of the boiling point elevation is directly proportional to the molality of the solution.
Formula used for Elevation in boiling point :
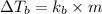
or,
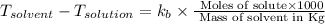
where,
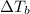 = change in boiling point
= change in boiling point
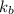 = boiling point constant
= boiling point constant
m = molality
Hence, the correct option is, (B) molality of solution