Answer:
The correct answer is 4 electrons.
Step-by-step explanation:
Reduction reaction is defined as chemical reaction in which element gains electrons and it get reduced. Also oxidation state of that element also deceases.
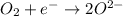
Charge on both sides should be balanced so for that write 4 as a coefficient of en electron in an above reaction.By this the charge on booth sides becomes (4-).

1 mole of oxygen gas gets reduced by 4 electrons into 2 moles of oxygen ions.