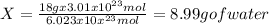Answer:
The answer is 8.99 g of water
Step-by-step explanation:
First we calculate the molecular mass of water, as follows. using the periodic table we have the masses of hydrogen and oxygen:
MW of H2 2 x 1 g/mol=2g/mol
MW of O: 16 g/mol
MW of H2O=2+16=18 g/mol
using Avogadro's number and making a simple direct rule of three we have:
6.023x
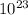 mol----------------------18 g
mol----------------------18 g
3.01x
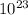 mol------------------------ Xg
mol------------------------ Xg
Clearing the X, we have: