Answer: The correct answer is Option A.
Step-by-step explanation:
There are 3 subatomic particles in an atom: Neutrons, electrons and protons.
Neutrons and protons reside in the nucleus of an atom.
Mass number of an atom is defied as the sum of number of neutrons and number of protons.
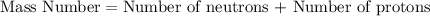
Thus, The sum of neutrons and protons will always be equal to the mass of an atom.
Hence, the correct answer is Option A.