there is a key piece of information that we are missing.
we need the following:
Kb of water= 0.512
the change in boiling point (ΔTb) can be calculated using the following formula:
ΔTb= Kb x m
we already have Kb, but we need to determine the molality (m).
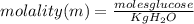
1) let's convert the grams of glucose to moles using the molar mass of it. The molecule formula of glucose is C₆H₁₂O₆.
molar mass C₆H₁₂O₆= (6 x 12.0) + (12 x 1.01) + (6 x 16.0)= 180 g/mol
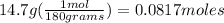
2) let's determine the Kilograms of water.
info:
density of water= 1.0 g/ mL or 1 grams = 1 mL
1000 grams= 1 kilogram
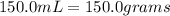

3) let's plug in the values to solve for molality
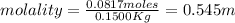
finally, we can solve for change in boiling point.
ΔTb= Kb x m
ΔTb= (0.512) (0.545m)=
0.279°C