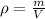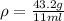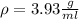Answer:
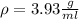
Step-by-step explanation:
Hi, when putting the stone in the water it moves a volume of water equal to its own volume. So, if the initial volume in the cylinder is 63 ml and the final is 74 ml, the stone's volume can be calculated as:
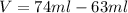
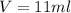
Now, you know the weigh and the volume of the philosopher's stone. The definition of density is mass per volume: