Answer: The correct answer is hydrogen ions.
Step-by-step explanation:
Acid-base indicator is used to detect the pH of the solution.
pH is defined as the negative logarithm of hydrogen ion concentration present in the solution.
- If the solution has high hydrogen ion concentration, then the pH will be low and the solution will be acidic. The pH range of acidic solution is 0 to 6.9
- If the solution has low hydrogen ion concentration, then the pH will be high and the solution will be basic. The pH range of basic solution is 7.1 to 14
- The solution having pH equal to 7 is termed as neutral solution.
The equation representing the reaction between hydrogen ions and acid-base indicator follows:
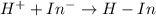
Hence, the correct answer is hydrogen ions.