Answer : The reactions that are exothermic have
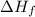 values that are negative.
values that are negative.
Explanation :
Exothermic reaction : When system releases heat energy to surrounding is known as exothermic reaction.
Endothermic reaction : When system absorbs heat energy from surrounding is known as endothermic reaction.
Enthalpy of reaction,
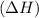 : It is defined as the heat energy changes when the reactants go to the products.
: It is defined as the heat energy changes when the reactants go to the products.
If the heat energy is released then the reaction is exothermic and the
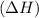 is negative that means product is more stable than the reactant.
is negative that means product is more stable than the reactant.
If the heat energy is absorbed then the reaction is endothermic and the
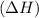 is positive that means product is less stable than the reactant.
is positive that means product is less stable than the reactant.
Hence, the reactions that are exothermic have
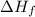 values that are negative.
values that are negative.