Hello!
A student requires 2.00 L of 0.100 M NH4NO3 from a 1.75 M NH4NO3 stock solution. What is the correct way to get the solution ?
Measure 114 mL of the 1.75 M solution, and dilute it to 1.00 L.
Measure 114 mL of the 1.75 M solution, and dilute it to 2.00 L.
Measure 8.75 mL of the 1.75 M solution, and dilute it to 2.00 L.
Measure 8.75 mL of the 0.100 M solution, and dilute it to 2.00 L.
We have the following data:
M1 (initial molarity) = 0.100 M (or mol/L)
V1 (initial volume) = 2.00 L
M2 (final molarity) = 1.75 M (or mol/L)
V2 (final volume) = ? (in L or mL)
Let's use the formula of dilution and molarity, so we have:
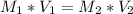
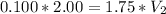
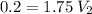
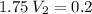
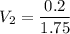
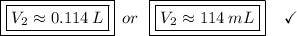
Answer:
Measure 114 mL of the 1.75 M solution, and dilute it to 2.00 L.
_______________________