Answer:
You add 75 ml of water to a volumetric flask, along with the 25 ml of stock solution, to prepare a 100 ml dilution
Step-by-step explanation:
Dilution is the procedure used to prepare a less concentrated solution from a more concentrated solution. It consists of adding solvent to an existing solution. Then the amount of solute does not vary, but the volume of the solvent does: when more solvent is added, the concentration of the solute decreases, as the volume of the solution increases.
In the case of a dilution, a Dilution Factor can be determined, which is a number that indicates how many times a solution must be diluted to obtain a lower concentration, such as:
Dilution factor =
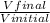
where Vfinal is the volume of the stock solution and Vinitial is the volume of the diluted solution.
In this case you need 25.0 ml of a stock solution to make 100.0 ml of a new diluted solution. So the dilution factor is:
Dilution factor=
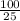
Dilution factor=4
This means that the initial solution must be diluted 4 times. Another way to see this is that for every 4 parts of this new diluted solution, 1 part is represented by the stock solution and 3 parts by the diluent water. Then, to prepare this solution, you must add 25.0 ml of stock solution to a volumetric flask, then add water until the total volume is equal to 100.0 ml.
So, you can do the calculation:
100 ml - 25 ml = 75 ml
This means that you add 75 ml of water to a volumetric flask, along with the 25 ml of stock solution, to prepare a 100 ml dilution. You diluted the stock solution by a factor of 4.